.png?width=4560&height=656&name=Website%20(8).png)
Research
Original, data-rich research that equips healthcare leaders with
authoritative insights into trends shaping the health economy.
authoritative insights into trends shaping the health economy.
%20(1).png?width=7120&height=5648&name=HPPT%20White%20Paper%20(3)%20(1).png)
Health Plan Price Transparency
Leveraging Transparency in Coverage Data to Reveal Actionable Information on Commercial Negotiated Rates
This report analyzes health plan price transparency data to examine inexplicable differences in healthcare prices.
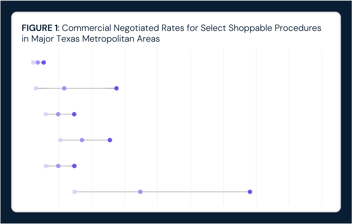
Intra-Market Rate Variation Can Lead to Spending Differences that Exceed $102M for a Single Orthopedic Procedure in Dallas-Fort Worth
Read More
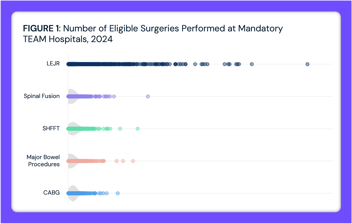
In the Same Market, Medicare Episodes of Care Can Range Up to 80x
Read More

AI in Healthcare: Current Uses, Shared Challenges and Future Stakeholder Opportunities
Read More

While Quality Is Relatively Comparable Across TEAM Hospitals, Financial Health Is Highly Variable
Read More
-2.png?width=354&height=224&name=Group%203%20(1)-2.png)
More Americans Are Dying at Home but Hospital-Based Deaths Remain Most Common
Read More
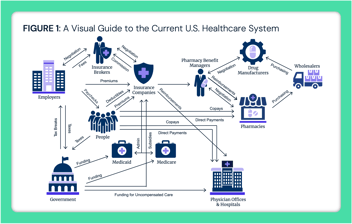
Middlemen in the U.S. Healthcare System: Shaping Costs and Complexity
Read More
.png?width=354&height=224&name=Group%201%20(14).png)
Primary Care Panels Vary in Size Based on Demographic Factors and Employment Structure
Read More

Potential Revenue Impacts of Elimination of the Medicare Inpatient Only List
Read More
Crunching the Numbers: Analysis of 2026 Healthcare Predictions
Read More








.png)

















.png?width=171&height=239&name=2025%20Trends%20Report%20Nav%20(1).png)
